Fuel Cells: The Complete Guide — Types, Working Principles, Applications & Comparisons
In the quest for a sustainable and decarbonized world, fuel cells have emerged as one of the most promising clean energy solutions. Unlike traditional combustion engines or fossil-fuel power plants, fuel cells generate electricity through an electrochemical reaction — producing only water and heat as by-products. This means zero local emissions and higher efficiency.
But did you know there are multiple types of fuel cells? Each type has unique working principles, temperature ranges, fuel requirements, and real-world applications — from powering laptops to buses, buildings, and even large-scale power plants.
In this comprehensive guide, we’ll explain how fuel cells work, break down the different types, show where they’re used, and compare them so you can see which is best for what purpose.
How Does a Fuel Cell Work?
At its core, a fuel cell converts chemical energy directly into electrical energy through an electrochemical reaction — similar to a battery, but it doesn’t run down or need recharging as long as fuel is supplied.
Basic working principle:
- Fuel (like hydrogen, methanol, or natural gas) is supplied to the anode side.
- Oxygen (from air) is supplied to the cathode side.
- At the anode, the fuel splits into electrons and protons.
- The electrolyte allows only the protons to pass through; the electrons flow through an external circuit, generating electricity.
- At the cathode, the protons, electrons, and oxygen combine to form water and release heat.
Each fuel cell type uses different fuels, electrolytes, and operating temperatures, which impact performance, cost, and application.
Types of Fuel Cells Explained in Detail
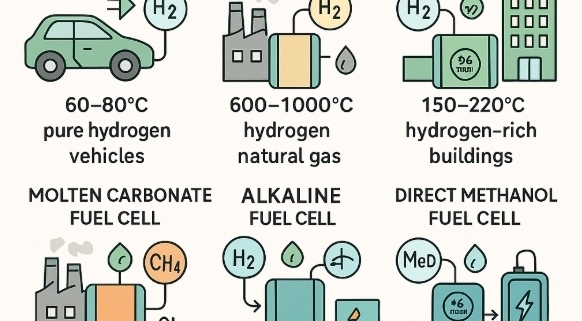
1. Proton Exchange Membrane Fuel Cell (PEMFC)
✅ How it Works:
Uses a solid polymer membrane as the electrolyte. Hydrogen fuel splits at the anode into protons and electrons; the membrane allows only protons through while electrons generate electricity via an external circuit. Operates at relatively low temperatures (~60–80°C).
✅ Typical Uses:
- Automobiles: hydrogen fuel cell cars (like Toyota Mirai, Hyundai NEXO)
- Buses & trucks
- Backup power for data centers and telecom towers
- Portable power packs
✅ Advantages:
- Fast start-up and shut-down
- Lightweight and compact
- Ideal for transportation
✅ Challenges:
- Requires pure hydrogen (sensitive to impurities)
- Expensive platinum catalyst needed
2. Solid Oxide Fuel Cell (SOFC)
✅ How it Works:
Uses a solid ceramic electrolyte that conducts oxygen ions. Operates at very high temperatures (600–1,000°C). Oxygen ions travel through the electrolyte to react with fuel (hydrogen or hydrocarbons) at the anode.
✅ Typical Uses:
- Large-scale stationary power generation
- Industrial combined heat and power (CHP)
- Distributed generation for commercial buildings
- Auxiliary power units for heavy-duty vehicles
✅ Advantages:
- High electrical efficiency (up to 60%)
- Can use various fuels: hydrogen, natural gas, biogas, syngas
- Waste heat can be used for CHP, increasing total system efficiency to ~80–90%
✅ Challenges:
- High operating temperature means long start-up times
- Expensive ceramic materials and sealing technologies needed
3. Phosphoric Acid Fuel Cell (PAFC)
✅ How it Works:
Uses liquid phosphoric acid as the electrolyte. Operates at moderate temperatures (~150–220°C). Oxygen is supplied to the cathode, while hydrogen-rich fuel reacts at the anode.
✅ Typical Uses:
- Commercial & industrial CHP
- Hospitals, hotels, and office buildings
- Distributed power generation where heat recovery is needed
✅ Advantages:
- Proven technology with commercial installations worldwide
- Good tolerance for fuel impurities
- Efficient cogeneration of heat and power (overall efficiency ~70–80%)
✅ Challenges:
- Lower electrical efficiency (~40–50%) than SOFC or PEMFC
- Bulky and heavy compared to newer fuel cell technologies
4. Molten Carbonate Fuel Cell (MCFC)
✅ How it Works:
Uses a molten carbonate salt mixture as the electrolyte, operating at around 600–700°C. Carbon dioxide and oxygen are fed to the cathode where carbonate ions are formed, migrating through the electrolyte to react with hydrogen at the anode.
✅ Typical Uses:
- Utility-scale power generation
- Large industrial facilities
- Industrial CHP systems
✅ Advantages:
- Can use carbon-based fuels like natural gas or biogas directly
- High electrical efficiency (~45–55%)
- Waste heat usable for industrial processes
✅ Challenges:
- High temperature requires durable materials and corrosion control
- Complex CO₂ management and system design
5. Alkaline Fuel Cell (AFC)
✅ How it Works:
Uses an alkaline electrolyte (potassium hydroxide solution) and operates at low to medium temperatures (~60–90°C). Very efficient at splitting hydrogen and oxygen.
✅ Typical Uses:
- Space missions (NASA’s Apollo and Space Shuttle used AFCs)
- Military applications
- Some portable or backup power solutions
✅ Advantages:
- High efficiency (up to 70% in some cases)
- Well-suited to pure hydrogen and oxygen environments
✅ Challenges:
- Sensitive to CO₂ contamination — needs purified hydrogen and air
- Limited commercial use outside niche applications
6. Direct Methanol Fuel Cell (DMFC)
✅ How it Works:
Uses a polymer electrolyte but runs directly on liquid methanol, eliminating the need for a fuel reformer. Methanol is oxidized at the anode to produce protons, electrons, and CO₂.
✅ Typical Uses:
- Small portable electronics (laptops, military field equipment)
- Backup power for telecoms
- Remote monitoring stations
✅ Advantages:
- Easy fuel storage and handling (liquid methanol)
- Simpler system design compared to hydrogen-based fuel cells
✅ Challenges:
- Lower efficiency than hydrogen fuel cells
- Methanol is toxic and flammable — needs careful handling
7. Reversible Fuel Cell (RFC)
✅ How it Works:
Also known as regenerative fuel cells, these operate as both electrolyzers and fuel cells. In electrolyzer mode, they use surplus renewable electricity to split water into hydrogen and oxygen for storage. When electricity is needed, they operate as a fuel cell to convert stored hydrogen back into power.
✅ Typical Uses:
- Renewable energy storage in microgrids
- Off-grid or remote systems with variable energy supply
- Long-duration storage solutions for excess solar/wind energy
✅ Advantages:
- Combines hydrogen production and power generation in one unit
- Ideal for integrating intermittent renewables
✅ Challenges:
- Still emerging — efficiencies, cost, and durability need improvement
- Complex system management to switch between modes
Comparison of Fuel Cell Types
| Fuel Cell Type | Operating Temp | Electrolyte | Typical Fuel | Best For | Electrical Efficiency |
|---|---|---|---|---|---|
| PEMFC | 60–80°C | Polymer membrane | Pure hydrogen | Cars, buses, backup power | 40–60% |
| SOFC | 600–1,000°C | Solid ceramic | Hydrogen, natural gas | Large CHP, industrial | 50–60% |
| PAFC | 150–220°C | Phosphoric acid | Hydrogen-rich | Buildings, CHP | 40–50% |
| MCFC | 600–700°C | Molten carbonate salt | Natural gas, biogas | Utility power, CHP | 45–55% |
| AFC | 60–90°C | Alkaline solution | Pure hydrogen | Space, niche portable | 50–70% |
| DMFC | 20–90°C | Polymer membrane | Liquid methanol | Portable power | 20–30% |
| RFC | Varies | Various | Water & hydrogen | Renewable storage | ~40–50% (emerging) |
Benefits and Challenges: A Quick Recap
✅ Benefits:
- Zero local emissions (only water, heat, and some CO₂ for carbon-based fuels)
- High fuel-to-electricity efficiency
- Quiet operation
- Scalable from milliwatts to megawatts
- Compatible with renewable hydrogen production
⚡ Challenges:
- Hydrogen infrastructure gaps (production, transport, storage)
- Catalyst costs (especially platinum for PEMFC)
- Durability and materials for high-temp systems
- System complexity for reversible and hybrid applications
Final Thoughts
From zero-emission vehicles to backup power for hospitals and large industrial plants, fuel cells offer versatile, reliable, and scalable clean energy solutions. As green hydrogen production and fuel cell technologies advance, we can expect to see these systems powering more of our daily lives.
🌍 Fuel cells aren’t just the future — they’re here now, transforming transportation, industry, and our energy grids.